Angelo lived an active life in the suburbs with his wife Courtney and their two kids, working at a nearby chemical plant for 30 years and finding any opportunity to take his family skiing. His life changed when he took a fall during a backyard volleyball game and his busy lifestyle went on pause. Angelo went in for an X-ray which revealed a lesion in his pelvis. A biopsy resulted in a devastating diagnosis—the 54-year–old New Jersey native had multiple myeloma, an incurable blood cancer that originates in plasma cells (a type of white blood cell) in the bone marrow.
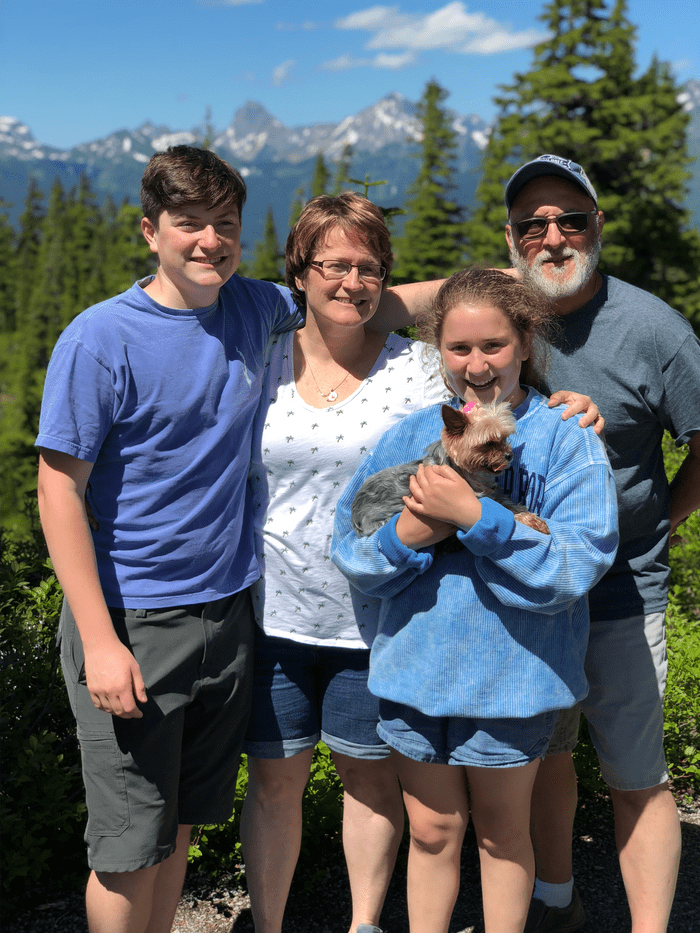
Despite advances in treatment, multiple myeloma is characterized by periods of remission (decrease in or no evidence of cancer) and relapse (when the cancer returns). There are several ways to treat multiple myeloma, such as chemotherapy, immunotherapy, and stem cell transplant. Each therapy comes with its own challenges and may require regular visits with a healthcare team, which can take a toll on patients. Additionally, as patients relapse and/or their disease becomes refractory (does not respond) to these therapies, fewer treatments remain as options.
For years, Angelo cycled through chemotherapy, immunotherapy, and two stem cell transplants to treat his multiple myeloma. “During that time in the hospital, there were times where I felt lousy,” Angelo explains. “I didn’t want to be there, and my head was just not in the right place.”
His doctors then suggested an innovative treatment approach called “chimeric antigen receptor T cell therapy,” commonly known as CAR T cell therapy. In this therapy, T cells, another type of white blood cell in the immune system, are genetically “reprogrammed” to recognize and fight cancer cells in the blood. After hearing about this treatment option, Angelo felt hopeful about the next step in his journey, and he received the CAR T cell therapy ABECMA®. ABECMA (idecabtagene vicleucel) is a prescription medicine for the treatment of multiple myeloma in patients who have received at least two kinds of treatment regimens that have not worked or have stopped working.
Please see full Prescribing Information, including Boxed WARNINGS and Medication Guide and Important Safety Information below.

After treatment, he spent two and a half weeks in the hospital, during which his care team monitored him for serious side effects like cytokine release syndrome (CRS), a condition where a large amount of proteins called cytokines are released into the blood, and neurologic toxicities (NT). Soon, his doctor shared the news he’d been hoping to hear: his cancer responded to the treatment.
Overcoming Hesitation and Embracing Innovation
Similar to Angelo’s journey, Bill, a 75-year-old retired helicopter pilot from Tennessee, and Cathrine, a 69-year-old former school bus driver, struggled with their multiple myeloma despite years of treatment. In addition to cycling through rounds of chemotherapy and other treatments, Bill underwent one stem cell transplant and Cathrine underwent two.
“When it comes to cancer, you need to put a lot of faith into what you’re trying to accomplish. You can’t give up,” says Bill. Encouraged by his doctor and family, he decided to receive ABECMA and his disease responded well to the treatment. “We put our faith in CAR T cell therapy, and it worked out well for us.” While Bill is no longer responding to treatment, he is grateful for the positive impact ABECMA provided in his treatment journey.
ABECMA is a one-time infusion, but the entire ABECMA treatment process can take two to three months from start to finish. It begins with a blood draw that separates T cells from other blood components. The T cells are sent to a specialized facility where they are genetically reprogrammed to become CAR T cells by adding tiny hooks, called chimeric antigen receptors (CAR), to their surfaces, which help target the cancer cells, as well as some normal cells, in the blood. The lab then reproduces millions of these CAR T cells to create the patient’s recommended dose of ABECMA.
Prior to receiving ABECMA, patients are given three days of chemotherapy to prepare the body for the infusion. After receiving ABECMA, the care team closely monitors for and manages CRS, NTs, and common side effects, including fatigue, fever, chills or shivering, severe nausea or diarrhea, decreased appetite, headache, dizziness or lightheadedness, confusion, trouble speaking or slurred speech, coughing, trouble breathing, and fast or irregular heartbeat.
Please see full safety information for ABECMA below.
“I knew that CAR T was an option and had side effects like other treatment options, but I was ready to try something different,” says Cathrine. “I was still a little bit scared but I focused on my grandkids – they motivate me to keep going.”
Cathrine worked closely with her care team to understand the possible side effects and how they could manage them, which helped alleviate her worries. After the ABECMA infusion, Cathrine experienced a fever and decrease in blood pressure, but her care team monitored and managed her symptoms closely in the hospital for about a week. Shortly after receiving ABECMA, Cathrine achieved a response to the therapy.
More About ABECMA
Angelo, Bill, and Cathrine received ABECMA after four or more lines of therapy did not work for them. However, ABECMA is now available in earlier lines of treatment, based on the most recent trial evaluating 386 people with relapsed or refractory multiple myeloma (RRMM) who received at least two prior medicines and were a median age of 63 years old. Those who received ABECMA (254 people) lived three times longer without their multiple myeloma coming back or progressing compared to standard treatment (132 people) (13.8 months vs. 4.4 months). Across two ABECMA clinical trials, 82% (287/349) of people had mild or moderate CRS, 7% (23/439) had severe or life-threatening CRS, including 3 people who had CRS leading to death; 34.9% (122/349) had mild or moderate neurologic side effects, and 4.9% (17/349) had severe or life-threatening neurologic side effects, including 1 person who had a neurologic side effect leading to death.
These trial results demonstrate how ABECMA can help patients in earlier lines of therapy fight against their multiple myeloma.
Hope After ABECMA
Like any chronic illness, multiple myeloma has a lifelong impact on patients, both physically and mentally. The impact can be felt in daily activities such as work, exercise, chores, and hobbies, all the way to meaningful moments like spending time with family and traveling. The prospect of having another treatment option like ABECMA gives patients hope to manage their disease and return to their day-to-day activities while responding to therapy.
Bill found a new normal and appreciation for life with the support of his wife. “We get up about quarter to seven, have breakfast, and then usually five or six days a week, I slip out with my buds and we solve a few of the world’s problems,” jokes Bill. “I’m not golfing or working out as much as I used to, but I have a peddler that I get 2,500 steps on in a day.”
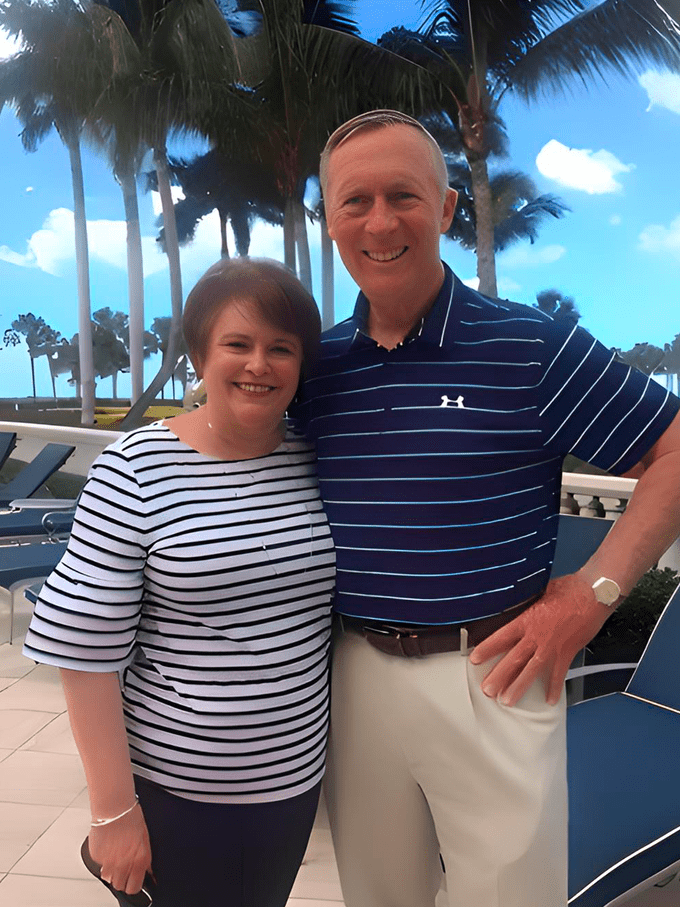
Since being treated with ABECMA, Cathrine has started working again part-time in a local elementary school cafeteria. She’s also been traveling with her family of 17, most recently to Hawaii and on a cruise through the Dominican Republic and U.S. Virgin Islands, and hopes to visit Rome again. “This treatment was the beginning of the next step of my journey,” Cathrine shares.
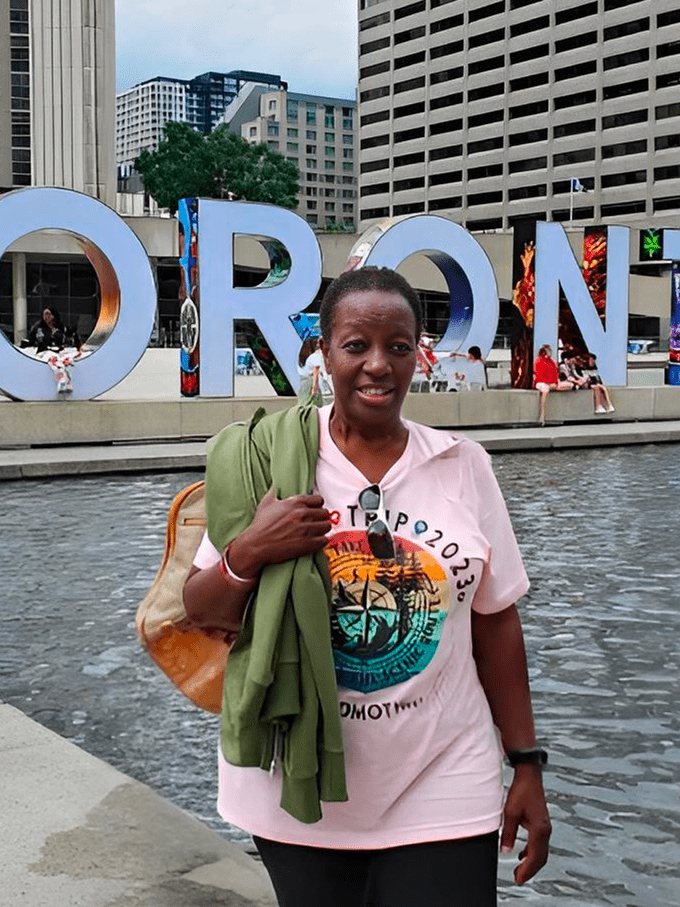
Angelo continues to be optimistic about his future, and after being monitored for months after treatment with ABECMA, he has slowly picked back up on his hobbies. “I’m pretty active and love to ski with my family and ride my motorcycle. I have to do what I like because when I stop doing the things I love, that’s when I know I’m getting old.”
Visit ABECMA.com to hear from other patients who have received ABECMA and to learn more about if this CAR T cell therapy option could be the next step in your multiple myeloma journey.
These are individual patient experiences. Talk to your doctor to determine if ABECMA is right for you. Please see Important Safety Information below, including potential side effects.
Important Safety Information
What is the most important information I should know about ABECMA?
ABECMA may cause side effects that are severe or life-threatening and can lead to death. Call your healthcare provider or get emergency help right away if you get any of the following:
- difficulty breathing
- fever (100.4°F/38°C or higher)
- chills/shivering
- confusion
- dizziness or lightheadedness
- shaking or twitching (tremor)
- fast or irregular heartbeat
- severe fatigue
- severe nausea, vomiting, diarrhea
It is important that you tell your healthcare providers that you have received ABECMA and to show them your ABECMA Patient Wallet Card. Your healthcare provider may give you other medicines to treat your side effects.
How will I receive ABECMA?
- ABECMA is made from your own white blood cells, so your blood will be collected by a process called “leukapheresis”.
- Your blood cells will be sent to a manufacturing center to make your ABECMA. Based on clinical trial experience, it takes about 4 weeks from the time your cells are received at the manufacturing site and are available to be shipped back to your healthcare provider, but the time may vary.
- Before you get ABECMA, your healthcare provider will give you chemotherapy for 3 days to prepare your body.
- When your ABECMA is ready, your healthcare provider will give ABECMA to you through a catheter (tube) placed into your vein (intravenous infusion). Your dose of ABECMA may be given in one or more infusion bags. The infusion usually takes up to 30 minutes for each infusion bag.
- You will be monitored at the certified healthcare facility where you received your treatment daily for at least 7 days after the infusion.
- You should plan to stay within 2 hours of this location for at least 4 weeks after getting ABECMA. Your healthcare provider will check to see that your treatment is working and help you with any side effects that may occur.
What should I avoid after receiving ABECMA?
- Do not drive, operate heavy machinery, or do other activities that could be dangerous if you are not mentally alert, for at least 8 weeks after you get ABECMA. This is because the treatment can cause temporary memory and coordination problems, sleepiness, confusion, dizziness, and seizures.
- Do not donate blood, organs, tissues, or cells for transplantation.
What are the possible or reasonably likely side effects of ABECMA?
The most common side effects of ABECMA are:
- fatigue
- fever (100.4°F/38°C or higher)
- chills/shivering
- severe nausea or diarrhea
- decreased appetite
- headache
- dizziness/lightheadedness
- confusion
- difficulty speaking or slurred speech
- cough
- difficulty breathing
- fast or irregular heartbeat
In a study comparing ABECMA to standard regimen, a higher proportion of patients experienced death within the first 9 months from randomization in the ABECMA arm compared to the standard regimens arm. This higher rate of early death was mainly observed before receiving ABECMA with the main reason being progression of multiple myeloma. There was also an increase in the rate of death from adverse events after ABECMA.
ABECMA can cause a very common side effect called cytokine release syndrome, or CRS, which can be severe or fatal. Symptoms of CRS include fever, difficulty breathing, dizziness or light-headedness, nausea, headache, fast heartbeat, low blood pressure, or fatigue. Tell your healthcare provider right away if you develop fever or any of these other symptoms after receiving ABECMA.
ABECMA can increase the risk of life-threatening infections that may lead to death. Tell your healthcare provider right away if you develop fever, chills, or any signs or symptoms of an infection.
ABECMA can lower one or more types of your blood cells (red blood cells, white blood cells, or platelets), which may make you feel weak or tired or increase your risk of severe infection or bleeding. After treatment, your healthcare provider will test your blood to check for this. Tell your healthcare provider right away if you get a fever, are feeling tired, or have bruising or bleeding.
ABECMA may increase your risk of getting cancers including certain types of blood cancers. Your healthcare provider should monitor you for this.
Having ABECMA in your blood may cause a false-positive human immunodeficiency virus (HIV) test result by some commercial tests.
This is a summary of the most important safety information about ABECMA. These are not all the possible side effects of ABECMA. Call your doctor for medical advice about side effects. For more information, go to www.ABECMA.com or call 1-888-805-4555. You may report side effects to the FDA. Visit: http://www.fda.gov/medwatch, or call 1-800-FDA-1088.
Please see full Prescribing Information, including Boxed WARNINGS and Medication Guide.
© 2025 Bristol Myers Squibb. All rights reserved. 01/25. 2012-US-2400332.
ABECMA and the related logo are trademarks of Celgene Corporation, a Bristol Myers Squibb company. All other trademarks are the property of their respective owners.
